COMPOUNDED STERILE PREPARATIONS
Large Volume Parenteral (LVP) -or, simply, LVPs- are sterile preparations of 250 ml or greater that are administered parenterally. The most common LVPs are IV solutions compounded from a standard/base solution, such as 0.9% sodium chloride (known as NS, too), dextrose 5% in water (D5W), dextrose 5% in normal saline (D5NS), and Lactate Ringer (LR) solution.
These IV solutions can be administered as either a continuous infusion or a drip; a continuous infusion—also called a maintenance infusion, replacement infusion, or hydration infusion—typically consists of a base solution with additives; unlike continuous infusions, drip solutions are used to continuously deliver an IV medication to treat a specific medical condition.
Note that the most common volumes for LVPs are 250 ml, 500 ml, and 1000 ml, but BRAM-COR design can generate turnkey projects for any size, up to 5000 ml for bags or canister.
ABOUT CSP PRODUCTS
Maintaining the correct level of fluid and electrolytes within the body (homeostasis) is crucial for life. When some conditions influence normal fluid intake and output, the body may be at risk for dehydration.
The fast method of rehydration is through the injection of parenteral solutions into the body supply. The large majority of Compounded Sterile Preparations (CSPs) set by IV technicians are LVPs, large volume parenterals. Because these products are administered directly into the human blood supply, the solutions must possess certain chemical properties that render them safe for the patients. For example, the human blood plasma has a pH of 7,4 (slightly alkaline)and this value must be maintained for optimum health. In this regard, some facilities inject a buffer solution into the CSP, such as sterile sodium bicarbonate, to neutralize the pH and inhibit misaligned values.
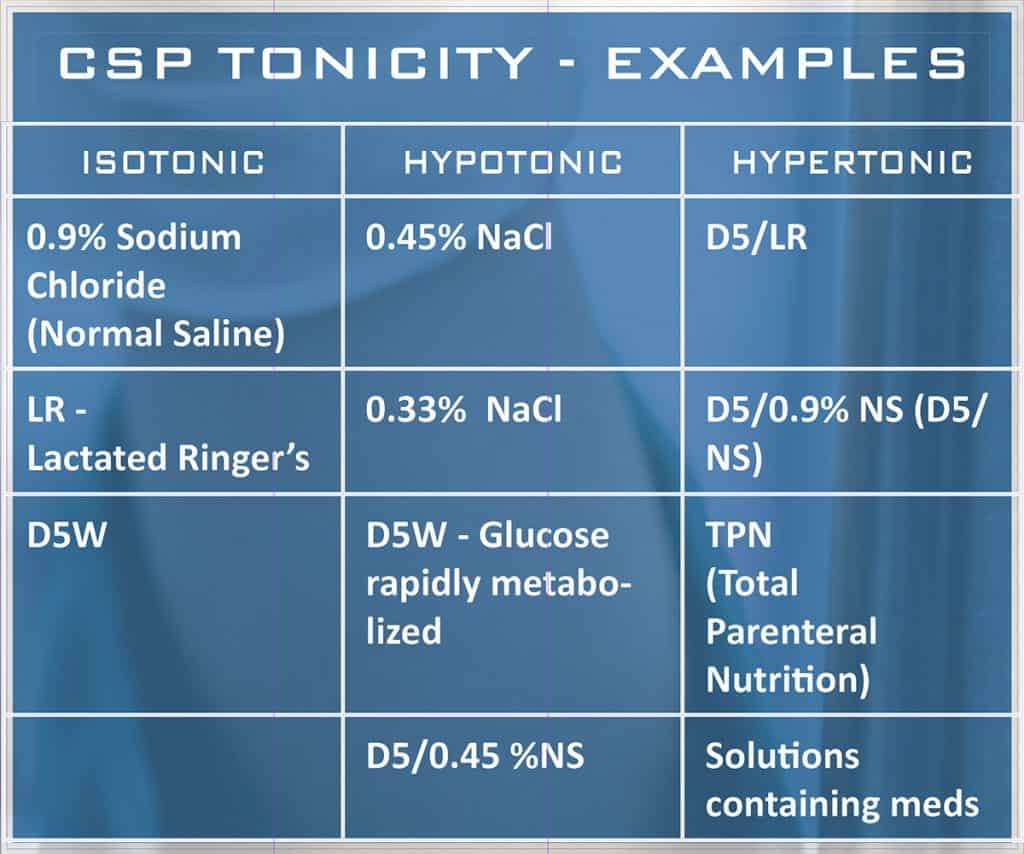
The isotonic property means that the CSPs have relatively the same number of dissolved particles and the same osmotic pressure as human blood plasma (conversely: Hypertonic solutions -tipically TPN- contain a greater number of dissolved particles; Hypotonic solutions contain fewer dissolved particles). In light of potential hazards, CSP preparations must be in adherence to USP Chapter <797> Guidelines (Pharmaceutical Compounding – Sterile Preparations) or other reference pharmacopoeias, as EP or JP.